Championing Change for Sickle Cell in California
Accelerating Research and Awareness of Sickle Cell Disease
The California Institute for Regenerative Medicine (CIRM) is funding regenerative medicine and gene therapy research to improve the lives of Sickle Cell patients in California and the world.
WHAT IS SICKLE CELL DISEASE?
Sickle cell disease is a genetic disorder that causes red blood cells to assume a sickle shape under stress – clogging blood vessels, producing episodes of excruciating pain called crises, and causing organ damage. This disease disproportionately affects people of African American heritage.
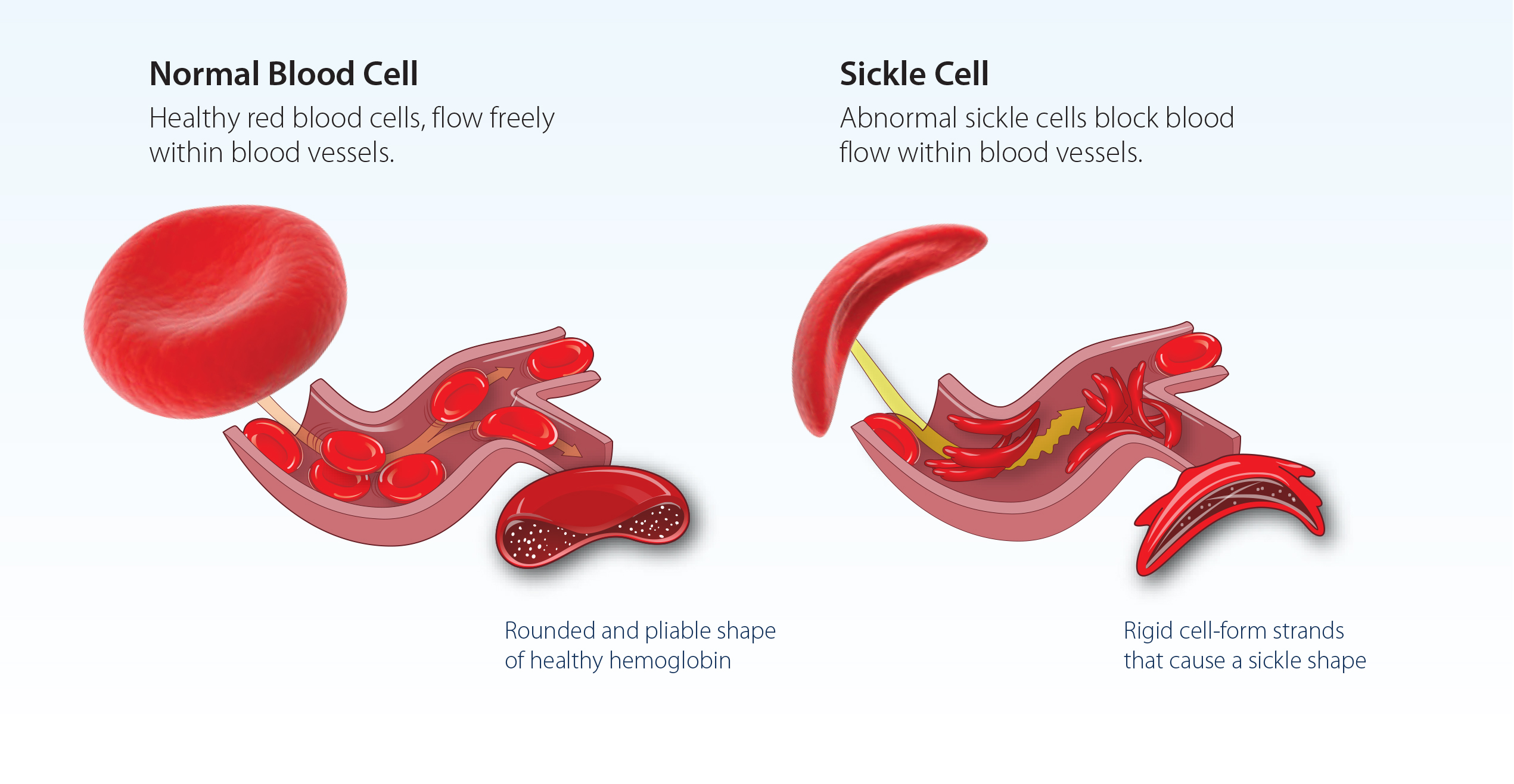
How is CIRM helping Sickle Cell patients?
CIRM is funding crucial research into stem cell, gene therapy, and other regenerative medicine procedures for sickle cell disease. We invest in many projects seeking to better understand the disease and translate those understandings into new therapies. To date, CIRM has invested over $65 million towards research for sickle cell—everything from basic discovery to clinical trials.
Our Mission for Sickle Cell
It is estimated that 100,000 Americans—7% of whom are from California—currently live with sickle-cell disease. In the last 30 years, the average life expectancy of an individual with sickle cell disease has dropped from 42 years to 39 years.
CIRM is committed to funding regenerative medicine research for sickle cell and helping spread education and awareness of this illness.
Funding Research is Crucial
CIRM accelerates research to fund future treatments for sickle cell disease. To date, CIRM has invested more than $65 million into research to help combat this illness.
Working Together to Champion Change
CIRM is accelerating cell and gene therapy research for sickle cell, but we can’t do it by ourselves. Community partners and patient advocates are all important to drive progress for sickle cell treatments.
This is why CIRM has partnered with key organizations such as sickle cell patient advocacy group Axis Advocacy, the United States’ National Heart, Lung, and Blood Institute’s (NHLBI) Cure Sickle Cell Initiative, and the UCSF Sickle Cell Center of Excellence. Through work with our partners, CIRM accelerates research and increases awareness of this debilitating condition across the state of California.
To help us aid sickle cell patients, learn how to sign up for one of Axis Advocacy’s patient advocacy programs.
Conducting Clinical-stage Research to Learn More
CIRM and NHLBI are co-funding two clinical trials seeking different types of gene therapy approaches for patients with severe sickle cell disease. Those include a Phase 1/2 trial led by Mark Walters, MD of UCSF Benioff Children’s Hospital Oakland and a Phase 2 trial led by David A. Williams, MD of Boston Children’s Hospital.
Clinical trials like these are instrumental to find new therapies for sickle cell. These crucial trials would not be possible without the help of everyday heroes who put their faith in the power of regenerative medicine and in CIRM.
Watch the story of Evie, one such hero and sickle-cell patient is who helping pioneer the future of medicine by participating in a CIRM-funded clinical trial at UCLA.
Progress in Sickle Cell Research
In 2023, the FDA approved two gene therapy treatments for sickle cell—Vertex and CRISPR Therapeutics’ Casgevy and bluebird bio’s Lyfgenia—bringing forth a new era of treatment options for people living with the condition. CIRM recognizes that more research and long-term innovation is needed to lower the costs of gene therapies, reduce the burden of the treatment on the patient, and make sickle cell therapies more accessible.

More Ways For You to Champion Sickle Cell Change.
Additional Resources
Download flyers and postcards to share with your community.
Check out these agencies and campaigns to learn more about Sickle Cell Disease.
Keep Up-To-Date
Sign up for our newsletter and stay up to date on new regenerative medicine events and research from CIRM.

















