Amyotrophic Lateral Sclerosis (ALS) Fact Sheet
CIRM funds many projects seeking to better understand ALS and to translate those discoveries into new therapies.
Description
About 6,000 people are diagnosed with ALS (also known as Lou Gehrig’s disease) each year in the U.S., and the average survival time is two to five years. The disease results when the cells in the brain or spinal cord that instruct muscles to move—called motor neurons—die off. People with the disease lose the ability to move their muscles and, over time, the muscles atrophy and people become paralyzed and eventually die. There is no effective therapy for the disease.
California’s stem cell agency has funded several research projects that could help people with ALS (the full list of CIRM awards in this disease is below). Some of those projects are very basic—researchers are trying to understand the origin of the disease and what causes the motor neurons to die. These are the kinds of questions researchers need to understand if they are going to develop the most effective therapies.
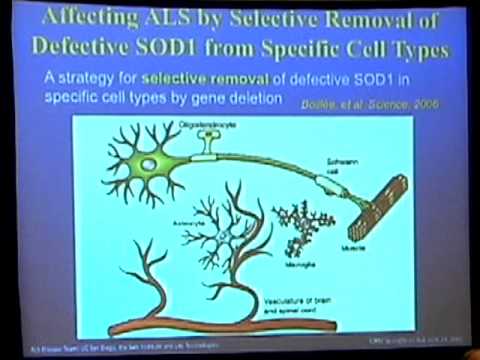
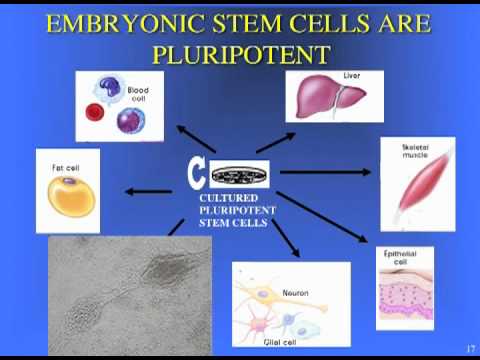
With CIRM funding, researchers have made progress understanding which cells are responsible for damaging the motor neurons. It turns out that the cells surrounding those neurons—called astrocytes—are secreting a chemical that damages the neurons. They’ve also learned how to take certain kinds of stem cells and turn them into motor neurons and astrocytes and this might help us better understand the relationship of these cells and even one day prove useful in developing new ways to treat people with ALS.
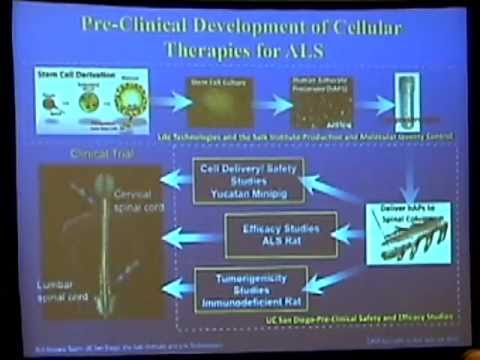
We also fund projects that are in the later stages of research leading up to and in some cases including clinical trials. These projects involve teams of researchers who carry out the experiments that are required before the U.S. Food and Drug Administration will allow the potential therapy to be tested in people. Recently, CIRM has funded research into ALS that has advanced into clinical trials. You can read more about these trials below.
Clinical Stage Programs
Cedars-Sinai Medical Center
This team of researchers plans to protect surviving neurons in people diagnosed with ALS from further degeneration. They will implant middle-man “progenitor” cells made by maturing stem cells from fetal tissue down a path destined to become astrocytes, the brain cells that protect nerves and that become defective in ALS. Those cells will be boosted with genes for a growth factor that when the cells release it after transplantation, will have an added protective effect on nerves. This approach recently received approval to treat ALS patients in a CIRM-funded clinical trial (read here). A feature story on this work appeared in The Stem Cellar blog in 2017.
Brainstorm Cell Therapeutics
BrainStorm is using mesenchymal stem cells that are taken from the patient’s own bone marrow to treat patients with ALS. These stem cells are then modified to boost their production of neurotrophic factors, which are known to help support and protect neurons, the cells destroyed by the disease. The CIRM funding will enable the company to test this therapy, called NurOwn, in a Phase 3 trial involving around 200 patients.
CIRM Grants Targeting ALS
| Researcher Name | Institution | Grant Title | Grant Type | Award Amount |
| Leif Havton | University of California, Los Angeles | Development of a Relevant Pre-Clinical Animal Model as a Tool to Evaluate Human Stem Cell-Derived Replacement Therapies for Motor Neuron Injuries and Degenerative Diseases | Tools and Technologies III | $1,308,711 |
| Prof. John M. Ravits | University of California, San Diego | Cell Therapy for amyotrophic Lateral Sclerosis (ALS) -Testing the Limits: What should we use as preclinical standards of clinical trials? | Conference | $7,193 |
| Professor Clive Niels Svendsen | Cedars-Sinai Medical Center | California ALS Summit 2012 | Conference | $6,825 |
| Dr. Martina Wiedau-Pazos | University of California, Los Angeles | California ALS Summit 2011 | Conference | $13,300 |
| Dr. Steve M. Finkbeiner | Gladstone Institutes, J. David | THE 5TH ANNUAL CALIFORNIA ALS PAC10 AND RESEARCH NETWORK MEETING | Conference | $9,529 |
| Dr. Joshua M Stuart | University of California, Santa Cruz | Center of Excellence for Stem Cell Genomics – UCSC | Genomics Centers of Excellence Awards (R) | $3,999,083 |
| Dr. Michael P Snyder PhD | Stanford University | Center of Excellence for Stem Cell Genomics – Stanford | Genomics Centers of Excellence Awards (R) | $22,787,914 |
| Dr. Lawrence S. B. Goldstein | University of California, San Diego | Stem Cell-Derived Astrocyte Precursor Transplants in Amyotrophic Lateral Sclerosis | Early Translational from Disease Team Conversion | $4,139,754 |
| Dr. Steve M. Finkbeiner | Gladstone Institutes, J. David | Development of Novel Autophagy Inducers to Block the Progression of and Treat Amyotrophic Lateral Sclerosis (ALS) and Other Neurodegenerative Diseases | Early Translational IV | $2,049,053 |
| Gene Wei-Ming Yeo | University of California, San Diego | Stem cell models to analyze the role of mutated C9ORF72 in neurodegeneration | Basic Biology IV | $1,260,360 |
| Professor Clive Niels Svendsen | Cedars-Sinai Medical Center | Progenitor Cells Secreting GDNF for the Treatment of ALS | Disease Team Therapy Development – Research | $16,168,464 |
| Gene Wei-Ming Yeo | University of California, San Diego | Molecules to Correct Aberrant RNA Signature in Human Diseased Neurons | Early Translational III | $1,532,323 |
| Professor Clive Niels Svendsen | Cedars-Sinai Medical Center | Stem Cells Secreting GDNF for the Treatment of ALS | Disease Team Therapy Planning I | $63,487 |
| Gene Wei-Ming Yeo | University of California, San Diego | Neural and general splicing factors control self-renewal, neural survival and differentiation | Basic Biology III | $1,287,619 |
| Dr. Eric T. Ahrens | University of California, San Diego | Molecular Imaging for Stem Cell Science and Clinical Application | Research Leadership | $5,680,474 |
| Dr. Bin Chen | University of California, Santa Cruz | Molecular mechanisms of neural stem cell differentiation in the developing brain | New Faculty I | $2,147,592 |
| Dr. Ying Liu | University of California, San Diego | Generation of disease models for neurodegenerative disorders in hESCs by gene targeting | Tools and Technologies I | $709,829 |
| Dr. Jeremy Reiter | University of California, San Francisco | High throughput modeling of human neurodegenerative diseases in embryonic stem cells | New Faculty II | $2,259,092 |
| Dr. Jerome A. Zack Ph.D. | University of California, Los Angeles | Generation of clinical grade human iPS cells | New Cell Lines | $1,341,000 |
| Dr. Fred H Gage | Salk Institute for Biological Studies | Development of Induced Pluripotent Stem Cells for Modeling Human Disease | New Cell Lines | $1,737,720 |
| Dr Hans S Keirstead | University of California, Irvine | hESC-Derived Motor Neurons For the Treatment of Cervical Spinal Cord Injury | Comprehensive Grant | $2,158,445 |
| Dr. Fred H Gage | Salk Institute for Biological Studies | Molecular and Cellular Transitions from ES Cells to Mature Functioning Human Neurons | Comprehensive Grant | $2,749,293 |
| Dr. Benhai Zheng Dr. | University of California, San Diego | Genetic manipulation of human embryonic stem cells and its application in studying CNS development and repair | SEED Grant | $600,441 |
| Prof. Samuel Lawrence Pfaff | Salk Institute for Biological Studies | Gene regulatory mechanisms that control spinal neuron differentiation from hES cells. | SEED Grant | $704,543 |
| Dr. Bin Chen | University of California, Santa Cruz | In vitro differentiation of hESCs into corticospinal motor neurons | SEED Grant | $465,624 |
| Dr. Lawrence S. B. Goldstein | University of California, San Diego | Stem Cell-Derived Astrocyte Precursor Transplants in Amyotrophic Lateral Sclerosis | Disease Team Research I | $5,694,308 |
| Dr. Bennett G Novitch Ph.D. | University of California, Los Angeles | Molecular Characterization of hESC and hIPSC-Derived Spinal Motor Neurons | Basic Biology I | $1,228,278 |
| Albert La Spada Ph.D. | University of California, Irvine | Role of ataxin-3 polyadenylation site selection in ALS neuron toxicity and disease pathogenesis | Foundation – Discovery Stage Research Projects | $1,514,416 |
| Julia Kaye | Gladstone Institutes, J. David | Developing a Human Model of Sporadic ALS Using Machine Learning and Robotic Microscopy | Foundation – Discovery Stage Research Projects | $1,406,622 |
| Dr. Justin K Ichida | University of Southern California | Development of a VAV2 antisense oligonucleotide (ASO) treatment for ALS | Quest – Discovery Stage Research Projects | $2,072,560 |
| Dr. Ritchie Ho | Cedars-Sinai Medical Center | C9orf72 repeat expansion-tuned allelic suppression by CRISPRi as an ALS therapy | Quest – Discovery Stage Research Projects | $2,274,768 |
| Ziwei Huang | University of California, San Diego | Development of a new therapeutic for directing target specific stem cell migration and treatment | Quest – Discovery Stage Research Projects | $1,129,512 |
| Dr. Justin K Ichida | University of Southern California | Development of a SYF2 antisense oligonucleotide (ASO) treatment for ALS | Quest – Discovery Stage Research Projects | $222,300 |
| Mr. Samuel V. Alworth MS, MBA | AcuraStem Incorporated | Development of AS-241, an UNC13A Targeting Antisense Oligonucleotide (ASO) Treatment for ALS, for IND-enabling Studies | Therapeutic Translational Research Projects | $4,012,325 |
| Dr. Lawrence S. B. Goldstein | University of California, San Diego | Human Embryonic Stem Cell-Derived Neural Stem Cell Transplants in Amyotrophic Lateral Sclerosis | Therapeutic Translational Research Projects | $1,790,000 |
| Dr. Justin K Ichida | University of Southern California | The 7th Annual California ALS research network and PAC10 meeting | Conference II | $10,830 |
| Prof. John M. Ravits | University of California, San Diego | California ALS Research Summit 2016 | Conference II | $11,400 |
| Clive Svendsen | Cedars-Sinai Medical Center | CNS10-NPC-GDNF delivered into the motor cortex for the treatment of ALS | Clinical Trial Stage Projects | $11,990,372 |
| Dr. Ralph Kern | BrainStorm Cell Therapeutics | A Phase 3, Randomized, Placebo-controlled Multicenter Study to Evaluate Efficacy & Safety of Repeated Administrations of NurOwn® in Patients with ALS | Clinical Trial Stage Projects | $15,912,390 |
| Professor Clive Niels Svendsen | Cedars-Sinai Medical Center | Human Neural Progenitors Secreting Glial Cell Line-Derived Neurotrophic Factor (CNS10-NPC-GDNF) for the Treatment of Amyotrophic Lateral Sclerosis | Clinical Trial Stage Projects | $6,154,067 |
| Mr. Samuel V. Alworth MS, MBA | AcuraStem Incorporated | Manufacturing of AS-202, an Antisense oligonucleotides for a Phase 1/2 Clinical Trial for Amyotrophic Lateral Sclerosis | Late Stage Preclinical Projects | $0 |
| | | | Total:
$130,611,816.43 |
CIRM ALS Videos
News and Information
Resources
Find Out More:
Stem Cell FAQ | Stem Cell Videos | What We Fund